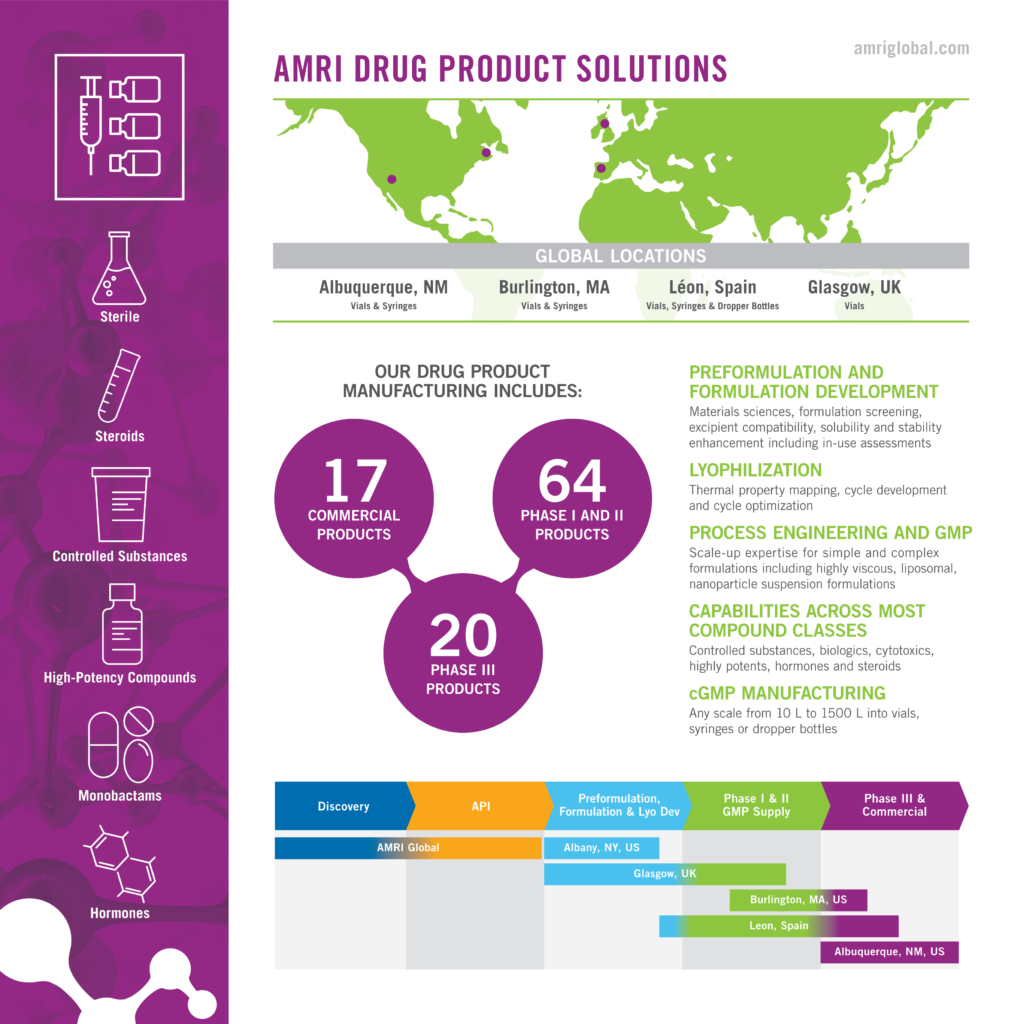
Optimize the Path From Bench to Market
Your project will benefit from our integrated sterile dosage form expertise, including complex liquid and lyophilized formulation development, scale-up and cGMP supply. Our extensive experience spans all compound categories for injectable, nasal and ophthalmic administration, and we offer final dosage form flexibility across vials, syringes or dropper bottles.
Access our services from four sterile GMP manufacturing facilities in Leon, Spain; Glasgow, U.K.; Burlington, Mass. (US) and Albuquerque, N.M. (US).