August 22, 2018
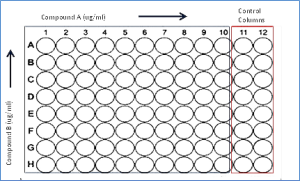
Test articles are first MIC tested against the strain selected to determine an appropriate range of test concentrations for the synergy test. Compounds are tested as nine (9) point, two-fold serial dilutions across the assay plate in combination with an seven (7) point, two-fold serial dilution of the second antibiotic (typically a standard of care antibiotic) down the assay plate. In addition each assay plate includes two (2) columns for control wells of uninhibited growth (no addition of test article) and for drug free sterile control wells (Figure 1). Assay plates are inoculated with 100 microliters of 5 x105 CFU/mL bacterial suspensions, incubated at 35°C for 16–24 hours and MIC values interpreted according to current CLSI breakpoints. Any clinical isolate from AMRI’s collection of aerobic strains may be used for the assay.
Figure 1. Checkerboard Assay Plate Layout
Fractional Inhibitory Concentration values (FICA and FICB) and FIC index are calculated for each combination of compounds and clinical isolate using the following standard equations for this analysis:
- FIC index = FICA + FICB
- FICA = MIC of compound A in combination / MIC of compound A alone
- FICB = MIC of compound B in combination / MIC of compound B alone
Synergy will be defined as a FIC index value of ≤0.5. Indifference or no interaction will be defined as a FIC index value of >0.5 and <4. Antagonism will be defined as a FIC index value of >4. When the FIC index value is within the range of 0.5 – 1, the combination is considered to be non-synergistic or additive. Results are provided in tabular form.
